A truly excellent clinician, podcaster, and researcher, Michael Ruscio, DC, recently focused his attention on Homocysteine. (You can read the transcript of his podcast here and stay tuned for an upcoming podcast of his in which we talk about it together!) I wanted to add to his discussion, and then asked myself: why haven’t I written about something I talk about every day in the office with my patients? No good answer, so here goes.
What is Homocysteine?
Homocysteine is an amino acid that our bodies make from methionine (which we absorb from eating animal protein) by removing a “methyl group.” Similarly, if you add a methyl group to homocysteine, we can recreate methionine and make that methyl group available to any other substance where methionine would like to donate it.
(Remember that we talked about methyl groups in the discussion on genetics. Just as French spend euros, and Americans exchange dollars, methyl groups are a widely useful currency in our human physiology. We transfer methyl groups from one substance to another, and thereby perform countless physiological functions. The estimate is millions of times a second, which is why I say countless: who can count that high?)
The big deal about homocysteine is that it seems to be essential, but in that Goldilocks way: too little can cause peripheral neuropathy and impair liver detoxification; too much is a risk factor for heart disease, blood clots, strokes, migraines, macular degeneration, hearing loss, pregnancy loss, neural tube defects, fractures, and neuropsychiatric illness, particularly memory loss.
High homocysteine and beta-amyloid overproduction are critical causative factors in Alzheimers Disease, although the mechanism is not completely understood. We are fairly certain that Alzheimer’s involves beta-amyloid overproduction, but also involves other factors: beta-amyloid alone is not sufficient to cause the disease.
Seems reasonable to avoid excess homocysteine, given a list of problems like that, but it’s more complicated than that.
The controversy
Homocysteine’s association with heart disease was noticed years ago. It was understood at the time that homocysteine gets too high when levels of folate (vitamin B9) and B12 are inadequate. Clinical experiments were performed in which people with high homocysteine were given folate as folic acid and B12 as cyanocobalamin. Homocysteine levels came down, but risk of heart disease wasn’t affected.
Two explanations are possible: the “normal values” for homocysteine are too lenient, currently set at 4-17 µmol/L. Many physicians (not all) believe that the optimal levels are between 5-8 µmol/L, and would consider anything over 10 definitely high. So perhaps they didn’t bring it down far enough.
The other possibility is that homocysteine isn’t the direct cause of heart disease. It can correlate with heart disease, without causing it, an important distinction to make in clinical medicine.
But that doesn’t mean we can forget about it.
Tweaking homocysteine
The body has many uses for homocysteine and many ways to keep it in its own sweet spot. When it is too low, it is not being replenished. When it is too high, it is not flowing smoothly out one of its normal pathways.
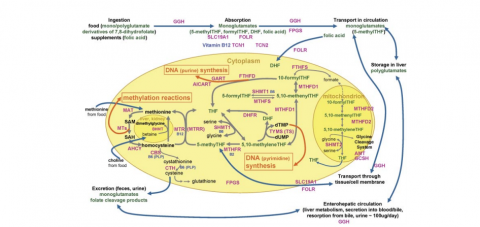
What are those pathways? I have to include a diagram—see the illustration above—without it I can only remember part of the picture.
The most widely understood mechanism for homocysteine accumulation is an inadequate supply of methyl folate (5-MTHF in the diagram) and methyl B12. We usually take those in to our body as plain folate, dihydrofolate or plain B12, from leafy greens, organ meat, and animal protein. Our body receives the folate and B12, and then attaches the methyl group. When our ability to methylate is inhibited, the active or methylated forms of the vitamins are in short supply and homocysteine accumulates. You can flood the body with folic acid and plain B12 and a certain percentage of those nutrients will be methylated will drive the homocysteine down, but if you have methylation issues you will also accumulate lots of unmethylated folate and B12 which will back up other necessary pathways. So when we’re addressing a deficiency, reflected in an elevated homocysteine, it’s important to ask the right question. The question is whether the deficiency is in the vitamins per se or whether the deficiency is in the process of methylating those vitamins.
If you have a terrible defect at methylating, you wouldn’t appear alive and healthy as you do now! If you have a moderate one, you probably will not know until you’re older, when your body is losing its ability to triage. Up to a point, we can all prioritize, the body can use what methyl groups it is able to attach, while it lets some less important functions go by the wayside. At some point, a limited ability to methylate catches up with one, as more necessary methylation functions are not performed.
But a high homocysteine can also mean that one of the other exit pathways isn’t working well. Homocysteine can be “sulfurated” and moves on to cysteine (and eventually glutathione, our master anti-oxidant) with the help of vitamin B6, or its active form pyridoxal-5-phosphate. This route actually lowers homocysteine, so either dietary insufficiency or ramped up sulfuration can explain a low homocysteine. Glutathione is so necessary for healthy survival that a low homocysteine becomes risky primarily for this reason: the person is not able to generate enough cysteine, sulfate, and eventually glutathione.
The third route to normalize a high homocysteine is through a zinc-dependent route via an enzyme called BHMT. When BHMT functions well, one of its functions is to provide an alternate exit pathway for excess homocysteine. When it doesn't function well, it might be as simple as a zinc deficiency or as specific as a choline deficiency (liver and egg yolks, crucial for this enzyme and for brain function in general.)
(When our bodies have at least three mechanisms for keeping homocysteine levels in a certain range, it makes me more sure that keeping homocysteine in its sweet spot is of value to us.)
The BHMT recycling process also helps replenish our levels of betaine, a widely useful compound known to protect internal organs, improve vascular risk factors, and increase performance. Alternately called “tri-methyl glycine,” betaine is able to transfer methyl groups where they are needed, and as it hands off methyl groups gradually changes into the highly useful amino acids, glycine and serine.
Checking homocysteine
I now check homocysteine routinely at least twice in my patients. If they are in the optimal range on two values at least a year apart, I doubt that they have any significant problem with homocysteine.
If their homocysteine levels are low, I think that they are at risk of a variety of conditions resulting from one of homocysteine’s metabolic pathways, particularly the one that leads to the creation of glutathione. (Low homocysteine associated conditions include schizophrenia, autism, accelerated aging, hepatitis, hyperlipidemia, diabetes, AIDS and vulnerability to environmental toxins.)
Far more often, however, the problem encountered is a high homocysteine. At the same time I order homocysteine, I also request a vitamin B12 and folate level, as well as an MTHFR genetic test. (I prefer that patients be tested through 23andMe for a fuller genetic panel, but at the very least will order this if I’m worried about homocysteine. I write the order as Homocysteine: if > 8, check MTHFR.)
Adjusting your own homocysteine
Homocysteine levels can vary widely. The highest I’ve seen in my own patients was in the upper 20’s, and I have seen only a couple slightly low levels.
If your homocysteine is high, should you panic? Absolutely not, but I would suggest a reasoned approach to lowering your levels, aiming for a goal of homocysteine between 5-8 µmol/L. Supplementation with methyl B12, chewable or sublingual, and methyl folate, each at 1 mg daily, is a conservative place to start for most people. People with multiple medical problems would benefit from a full genetic test to identify which part of the homocysteine pathway needs augmentation as well as the safest way in which to do it.
